Abstract
Silicone rubber has very different properties from general synthetic rubber.
Physical properties
It has properties such as heat resistance and high electrical insulation. It does not harden even at low temperatures. It is resistant to ultraviolet rays, and physiologically inactive. These characteristics are due to the difference in silicone rubber’s molecular structure from general rubber.
Chemical properties
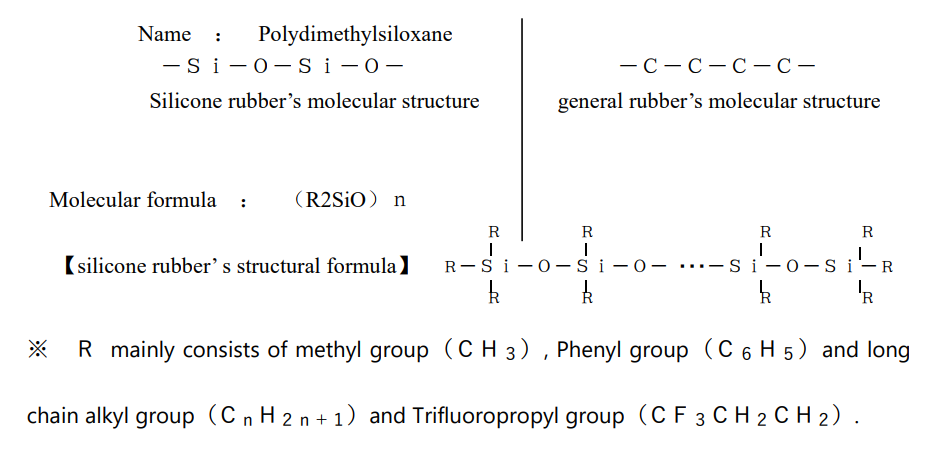
General rubber is a bond of carbon and carbon, but silicone rubber is a bond of silicon and oxygen. This bond is called a siloxane bond, and the characteristic properties of silicone rubber are due to this siloxane bond.
The binding energy of Si—O in silicone rubber is considerably larger than that of CC and CO bonds, so it is chemically stable. In addition, the Si—O bond has about 50% ionic bond property compared to the CC bond, which consists of 100% covalent bond, and can be said to be located between organic and inorganic. It is thought that the presence of this ionic bond strengthens the bond between CH of the methyl group which directly bonded to silicon and contributes to stabilization.